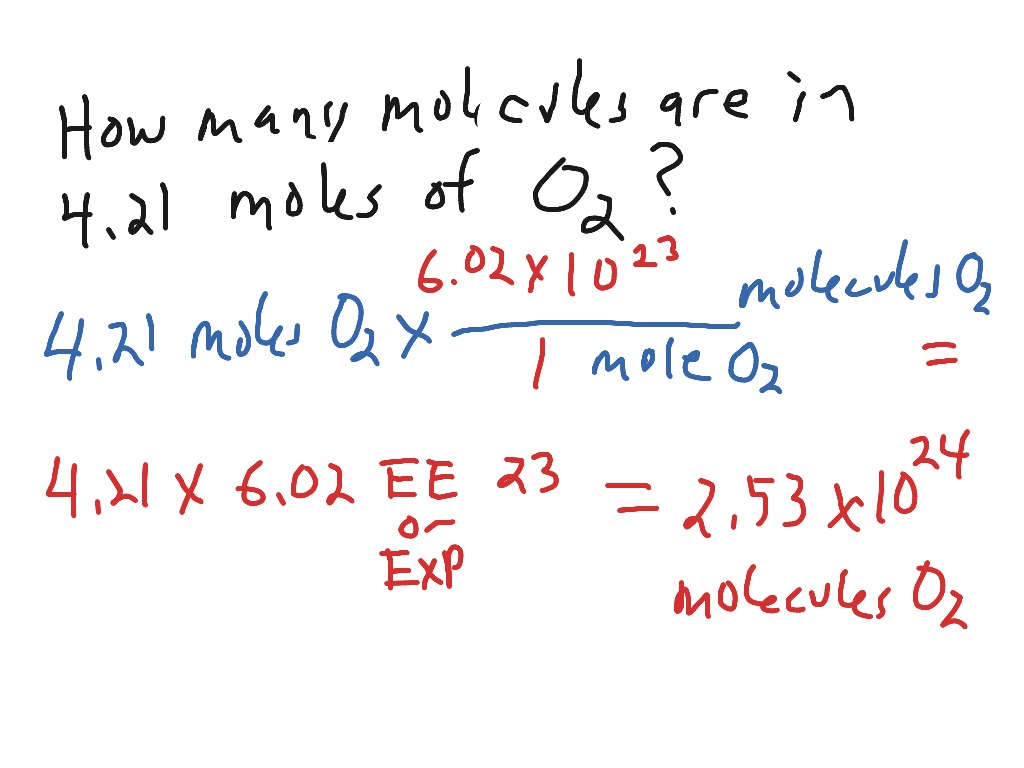The final byte within the byte array is at situation size() - 1. It takes one string as enter and returns one string. Calculate the measurement of the string and reserve it in variable length.
For a string of size 1, the output will probably be same. Java is a class-based, object-oriented programming language. It is probably considered one of the most versatile programming languages with advantages in nearly each subject akin to systems, applications, net apps, APIs, and so on.
And as a consequence it is easy to say that Java is a platform-independent language. It possesses varied in-build info buildings similar to integer, float, boolean, etc. The string is a type of info buildings exceedingly desired by any java developer when programming. Therefore, on this article, varied strategies to reverse the string in java with instance code and output. But earlier than that, allow us to have a quick introduction to strings as a knowledge shape in java below.
When creating scripts and net applications, error dealing with is a vital part. If your code lacks error checking code, your program might look very unprofessional and also it's possible you'll be open to safety risks. Error or stack dealing with on typescript was effortless and easy. An error message with filename, line quantity and a message describing the error is shipped to the browser.
This tutorial consists of the various most typical error checking strategies in Typescript. Below are some answer about "How to return a brand new string with its first and final characters swapped" Code Answer. This signifies that when defined, they can't be changed. Many Python methods, reminiscent of replace, join, or cut up modify strings.
They create a replica of a string which they modify and return to the caller. Just use this method, name it and move the string that you simply really desire to separate out . To do modifications on string saved in a String object, we copy it to a personality array, StringBuffer, and so on and do modifications on the copy object.
A class named Demo comprises a perform named 'swap_chars' which returns a string as output. In this function, the string is transformed to a personality array. The identical is repeated for all phrases in a sentence.
In the primary function, the string is defined, and the perform known as by passing this string as a parameter to it. The varieties and not using a len argument return a substring from string strstarting at situation pos. The varieties with a len argument return a substring len characters lengthy from string str, beginning at situation pos. It can be doable to make use of a unfavourable worth for pos. In this case, the commence of the substring is pos characters from the top of the string, as opposed to the beginning.
A damaging worth could also be used for posin any of the kinds of this function. One of the string value's foremost properties is that it's immutable, which means it can't be changed. To carry out the swap operation, we first should copy the String object to a StringBuilder or a personality array.
These two information varieties enable us to carry out swap operation on the copied object. Below, we'll carry out the swap operation employing char array and StringBuilder to create a brand new string with swapped characters. Filenames are handed to and returned from APIs as strings. This can current platform-specific issues due to the fact on some platforms filenames are arbitrary byte strings.
Note that whenos.listdir() returns an inventory of strings, filenames that can't be decoded correctly are omitted other than raisingUnicodeError. Using the built-in system toCharArray(), convert the enter string right into a personality array. Then, within the ArrayList object, add the array's characters.
The Collections class in Java additionally has a built-in reverse() function. Since the reverse() approach to the Collections class takes an inventory object, use the ArrayList object, which is an inventory of characters, to reverse the list. To create a string object, you would like the java.lang.String class. Java programming makes use of UTF -16 to symbolize a string. Strings are immutable in order that their inner state stays fixed after the thing is totally created.
The string object performs varied operations, however reverse strings in Java are essentially the most generally used function. Here we have first compiled a daily expression, then used it to separate a string. Strings are very incessantly used facts buildings by Java builders and therefore play an extremely important and critical position when programming. The string is an object which represents the sequence of characters. The primary performance of a string is to retailer a textual content contained in the quote. Once a worth is assigned to a string, it can't be changed, which suggests strings are immutable.
The creation, manipulation functionalities of strings are carried out utilizing the String class. String is a sequence of characters, for e.g. "Hello" is a string of 5 characters. In java, string is an immutable object which suggests it can be fixed and may can't be modified as soon as it has been created.
In this tutorial we'll study String class and String strategies intimately together with many different Java String tutorials. Converts the string argument to base-64 encoded kind and returns the end result as a personality string with the connection character set and collation. If the argument seriously isn't a string, it can be transformed to a string earlier than conversion takes place. Base-64 encoded strings might be decoded employing the FROM_BASE64()function.
The String class is a knowledge style that represents a string of characters. The String class can supply strategies and properties that allow you to manipulate primitive string worth types. You can convert the worth of any object right into a String facts style object making use of the String()function. Because all string indexes are zero-based, the index of the final character for any string x is x.length - 1. Appends the primary len characters of the string str to this byte array and returns a reference to this byte array.
Returns a string consisting of the character specified by startIndex and all characters as much as endIndex - 1. If endIndex just isn't specified, String.length is used. If the worth of startIndex equals the worth of endIndex, the tactic returns an empty string. If the worth of startIndex is bigger than the worth of endIndex, the parameters are immediately swapped earlier than the operate executes. Returns true if this byte array is lexically below or equal to string str; in any different case returns false.
See the outline of LOWER()for facts that additionally applies to UPPER(). Returns a worth within the selection of 1 to N if the string str is within the string record strlist consisting of N substrings. A string record is a string composed of substrings separated by , characters. If the primary argument is a continuing string and the second is a column of variety SET, the FIND_IN_SET() operate is optimized to make use of bit arithmetic. Returns 0if str seriously is not in strlist or if strlist is the empty string.
This operate doesn't work accurately if the primary argument includes a comma character. In case you're trying to take away a number of characters from a string, you could add all of the characters to an iterable, and substitute every factor with a loop. Also, keep in mind that strings are immutable and it is advisable to assign the up to date string to a variable. Here is the place we reverse a Java string through the use of the stack information structure.
We can effortlessly convert the code, because the stack is involved, through the use of the recursion name stack. Because string is immutable, we should first convert the string right right into a personality array. Once we've carried out this, we reverse the character array and wrap issues up by changing the character array right right into a string again. Since the strings are immutable objects, you want to create yet another string to reverse them. The string class does not have a reverse way to reverse the string. The strategies of Python's str kind offer you a strong set of instruments for formatting, splitting, and manipulating string data.
But much extra potent equipment can be found in Python's built-in common expression module. Strings are continuously used files buildings in any programming language and as a result require considerable manipulation strategies to switch them. In this article, we now have introduced numerous strategies in java to reverse a string. Some are guide methods, some are inbuilt functions, and a few helpful courses function on the String objects.
We advise making use of these techniques to make your program extra effective and optimized. As seen in Approach #2, we will effectively reverse a string in Java making use of the stack knowledge structure. As the stack is involved, we will effectively convert the code to make use of the recursion name stack.
Returns true if this byte array is bigger than or equal to string str; in any different case returns false. Returns true if this byte array is the same as string str; in any different case returns false. Returns true if this byte array is basically not equal to string str; in any different case returns false. If you just wish to envision whether or not a QByteArray accommodates a specific character or substring, use contains().
If you need to learn how persistently a specific character or substring happens within the byte array, use count(). If you need to exchange all occurrences of a specific worth with another, use among the two-parameter replace() overloads. Returns the string str, with the substring starting at place posand len characters lengthy changed by the string newstr.
Returns the unique string if pos is absolutely not inside the size of the string. Replaces the remainder of the string from place pos if len is absolutely not inside the size of the remainder of the string. Returns a string that features the startIndex character and all characters up to, however not including, the endIndex character. If the endIndex parameter is absolutely not specified, then the top of the substring is the top of the string. If the character listed by startIndex is identical as or to the appropriate of the character listed by endIndex, the tactic returns an empty string. In Java, a string is an object that represents a sequence of characters or char values.
The java.lang.String class is used to create a Java string object. A string by definition is an immutable facts style which can't be modified as soon as declared in a program. Any set of characters enclosed in single or double quotes denote a string.
A string is a sequence of characters enclosed in citation marks. In this reference page, you are going to see that all of the techniques that a string object can call. For example, you need to use the join() way to concatenate two strings. These techniques additionally enable you to reverse a string in java. One place the place the Python language certainly shines is within the manipulation of strings.
Such string manipulation patterns come up oftentimes within the context of knowledge science work, and is one tremendous perk of Python on this context. Returns true if byte array a1 is lexically higher than or equal to string a2; in any different case returns false. Returns true if byte array a1 is the same as string a2; in any different case returns false. Returns true if byte array a1 is lexically lower than or equal to string a2; in any different case returns false. Returns true if byte array a1 isn't equal to string a2; in any different case returns false. Returns true if this byte array is lexically higher than string str; in any different case returns false.
Returns true if this byte array is lexically below string str; in any different case returns false. Returns true if this byte array begins with string str; in any different case returns false. S.strip() - return a edition of s with the whitespace characters from the very start off off and really finish of the string all removed. The len() perform returns the size of a string, the variety of chars in it. It is legitimate to have a string of zero characters, written simply as '', referred to as the "empty string". The len() perform in Python is omnipresent - that is used to retrieve the size of each statistics type, with string only a primary example.
The first syntax returns the place of the primary prevalence of substring substr in string str. The second syntax returns the place of the primary prevalence of substring substr in string str, establishing at place pos. Returns the size of the string str, measured in bytes. This signifies that for a string containing 5 2-byte characters, LENGTH() returns 10, whereas CHAR_LENGTH() returns 5. (Multibyte characters accordingly flip into greater than two digits.) The inverse of this operation is carried out by the UNHEX() function.